TargetEpidermal growth factor receptor(Homo sapiens (Human))
Institute of Molecular Physiology
Curated by ChEMBL
Institute of Molecular Physiology
Curated by ChEMBL
TargetPotassium voltage-gated channel subfamily A member 3(Homo sapiens (Human))
Institute of Molecular Physiology
Curated by ChEMBL
Institute of Molecular Physiology
Curated by ChEMBL
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of voltage-gated Kv1.3 potassium channel (unknown origin) expressed in CHO cells by 86Rb efflux experimentMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Institute of Molecular Physiology
Curated by ChEMBL
Institute of Molecular Physiology
Curated by ChEMBL
Affinity DataIC50: 2.40E+3nMAssay Description:Inhibition of EGFRMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
Institute of Molecular Physiology
Curated by ChEMBL
Institute of Molecular Physiology
Curated by ChEMBL
Affinity DataIC50: 2.50E+3nMAssay Description:Inhibition of BTKMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily A member 3(Homo sapiens (Human))
Institute of Molecular Physiology
Curated by ChEMBL
Institute of Molecular Physiology
Curated by ChEMBL
Affinity DataIC50: 2.50E+3nMAssay Description:Inhibition of voltage-gated Kv1.3 potassium channel (unknown origin) expressed in CHO cells by 86Rb efflux experimentMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily A member 3(Homo sapiens (Human))
Institute of Molecular Physiology
Curated by ChEMBL
Institute of Molecular Physiology
Curated by ChEMBL
Affinity DataIC50: 3.70E+3nMAssay Description:Inhibition of voltage-gated Kv1.3 potassium channel (unknown origin) expressed in CHO cells by 86Rb efflux experimentMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily A member 3(Homo sapiens (Human))
Institute of Molecular Physiology
Curated by ChEMBL
Institute of Molecular Physiology
Curated by ChEMBL
Affinity DataIC50: 3.90E+3nMAssay Description:Antagonist activity at voltage-gated Kv1.3 potassium channel (unknown origin)More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2(Homo sapiens (Human))
Institute of Molecular Physiology
Curated by ChEMBL
Institute of Molecular Physiology
Curated by ChEMBL
Affinity DataIC50: 7.00E+3nMAssay Description:Inhibition of CDK2/Cyclin BMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2(Homo sapiens (Human))
Institute of Molecular Physiology
Curated by ChEMBL
Institute of Molecular Physiology
Curated by ChEMBL
Affinity DataIC50: 7.00E+3nMAssay Description:Inhibition of CDK2/Cyclin EMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 3(Homo sapiens (Human))
Institute of Molecular Physiology
Curated by ChEMBL
Institute of Molecular Physiology
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of ERK1/MAPKMore data for this Ligand-Target Pair
Affinity DataIC50: 6.40E+5nMAssay Description:Inhibition of IRKMore data for this Ligand-Target Pair

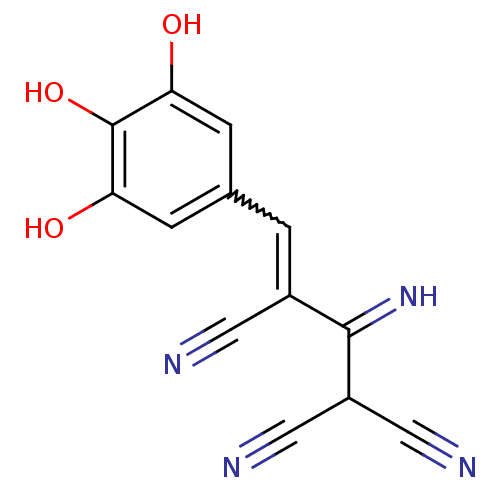
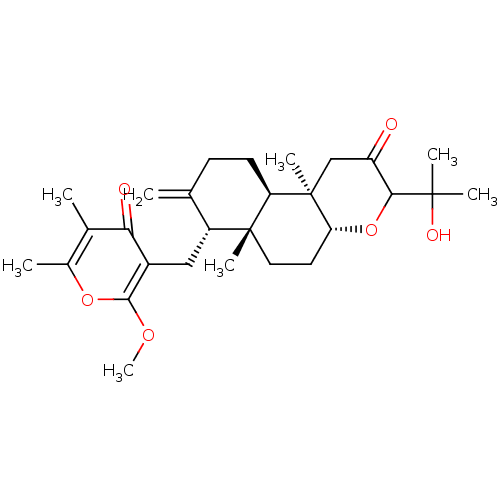
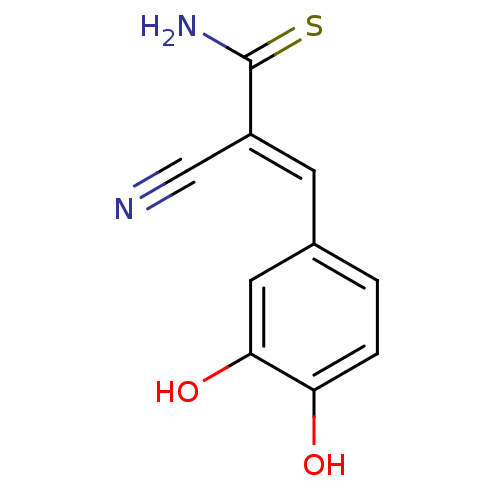

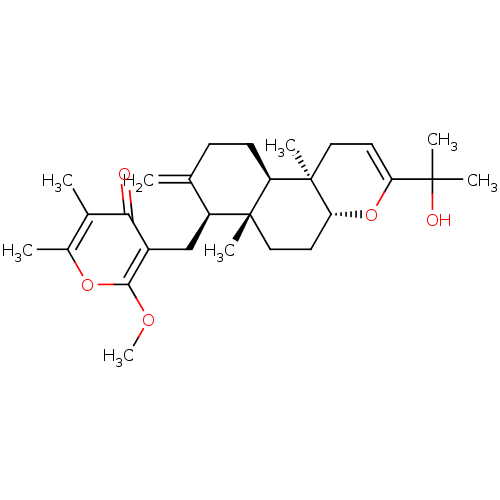
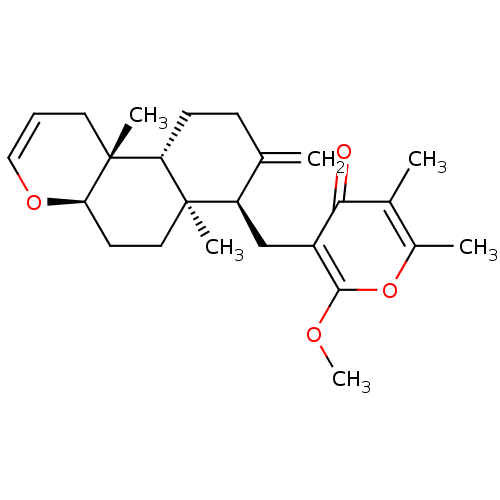
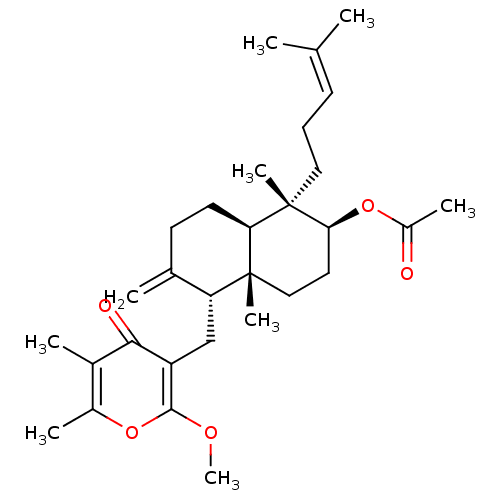
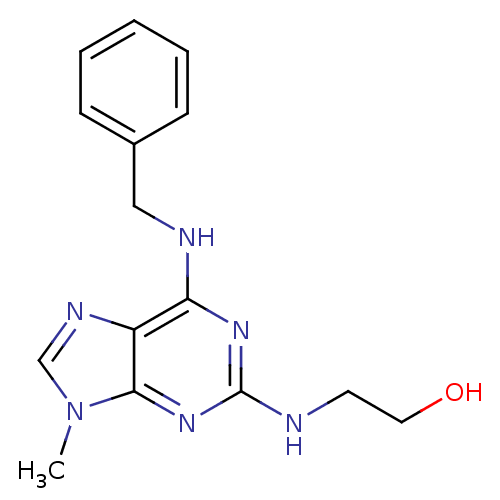
 3D Structure (crystal)
3D Structure (crystal)